An Immediate Stock Alert is Being Called On:
Algernon Pharmaceuticals Inc.
(OTC: AGNPF) (CSE: AGN)
Keep Reading Below to See Why…
While we’ve seen industries like food delivery, and even gold, benefit from the recent pandemic, no stocks have produced bigger gains for investors than coronavirus penny stocks dealing directly with treating and preventing the virus.
BREAKING NEWS: Algernon Announces Enrollment of 50th Patient in Multinational 2b/3 Human Study of Ifenprodil for Treatment of COVID-19
Here are just a few of the many big winners so far since the start of 2020:
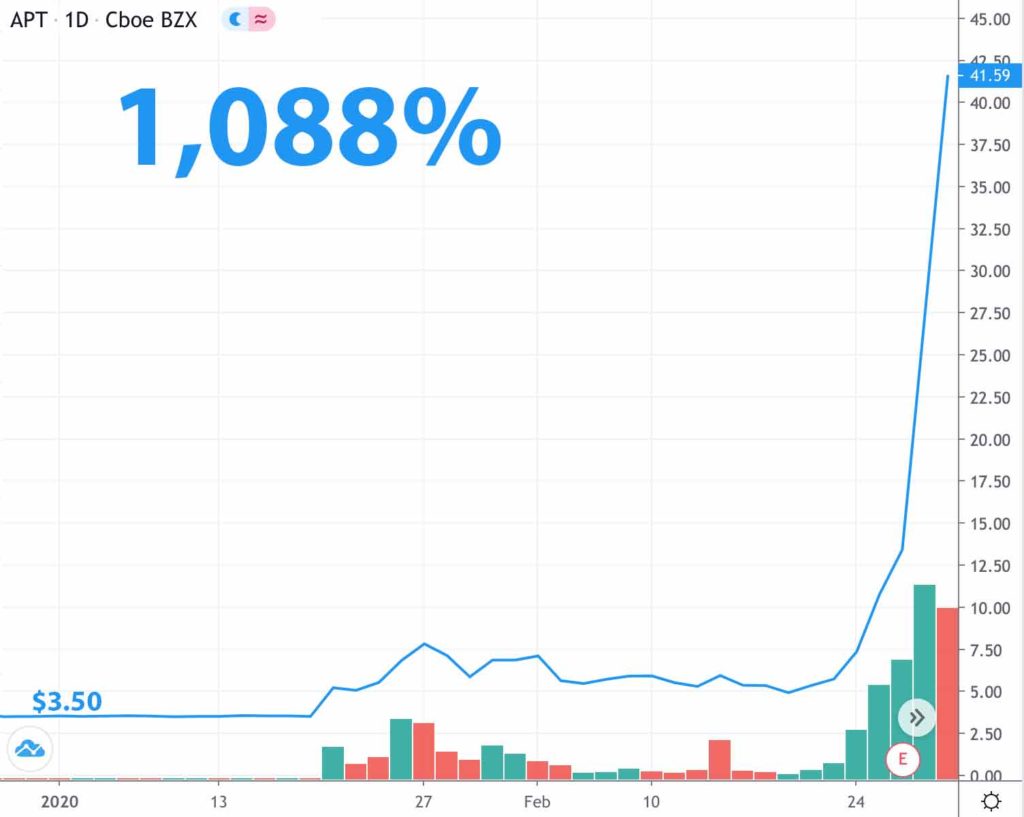
Alpha Pro Tech exploded nearly 1,100% when early investors figured out that COVID-19 might result in a need for facemasks; they were right!
AIM Immunotech shares skyrocketed over 1,100% when early investors began speculating on its influenza treatment for COVID-19
Allied Healthcare Products jumped 3,589% after early investors figured out that its respirator products could be useful during this pandemic
Novavax rallied over 4,600% once investors found out it had a compound that might treat COVID-19
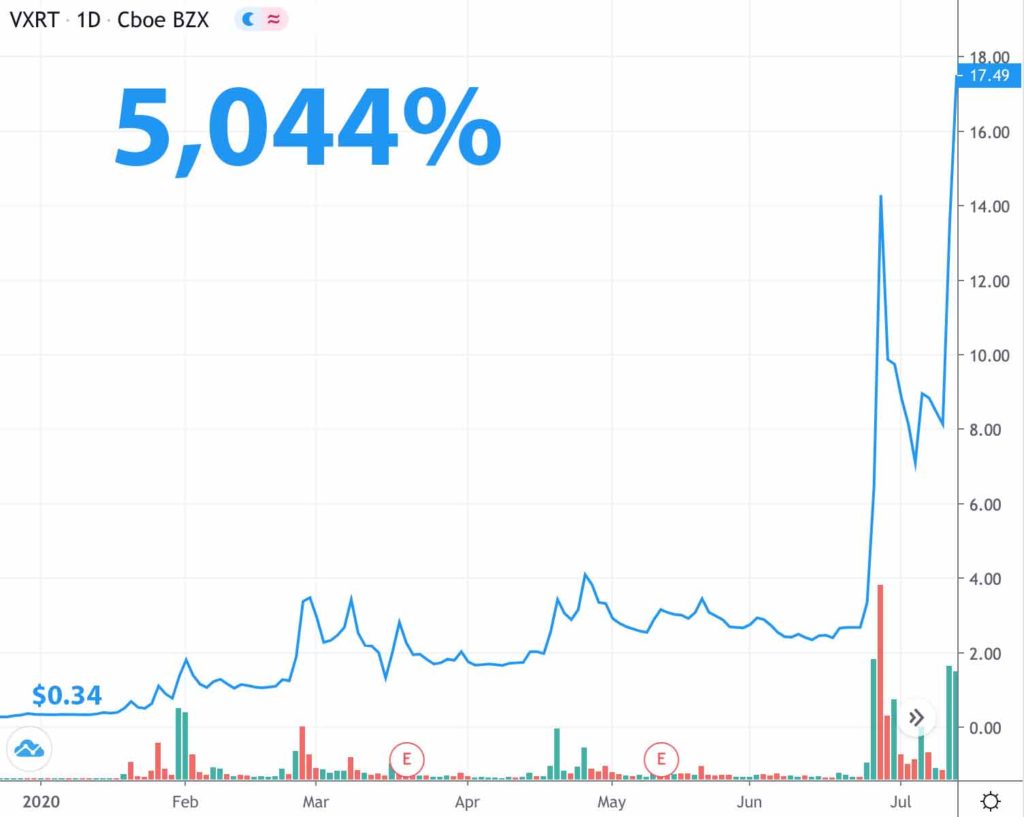
Vaxart took off more than 5,000% once investors found out it had a flu vaccine applicable to coronavirus.
This Could Be Just the Beginning for Coronavirus Stocks As Cases Continue To Climb!
What has been the significant factor for making money with coronavirus stocks? Being early. Notice that “early investors” in every case managed to reap the most rewards along the way. The fact that not one but multiple stocks (including many not mentioned here) produced thousands in percentage gains means that there is likely a huge focus on what’s next.
While the first wave of speculation taught us a lot and produced record-setting gains, the next wave of “undiscovered” COVID-19 names could deliver even more.
Again, the key is timing because as we’ve seen from previous pandemics, there’s an optimal window of time to take advantage of; now is that time.

(OTC: AGNPF) (CSE: AGN)
The coronavirus has created a mad panic across the world and by now billions of people not only know what it is but are also well-aware that the healthcare industry is coming together to work on a vaccine or to find a drug treatment.
[Read More] Best Penny Stocks to Invest in 2021? 3 For Your Watchlist
For investors, the next coronavirus stock to watch is a huge focus and when you talk about timing and opportunity in the market, getting your eyes on the next potential company to break out is key. Like we showed in the examples above, the ones who were early caught the most gains…to the tune of thousand of percentage points. The fact that this wasn’t just from “a couple lucky stocks” is even more of a reinforcement that this is where you need to be looking if your goal is profiting from this race for innovation.
The #1 Rule For Investing is: Buy Low & Sell High!
That couldn’t be more true than right now and the evidence suggests that investors are looking for the next “Buy Low” scenario. While all of the previous coronavirus stocks are trading much higher, are there any left to watch that hasn’t been discovered by the masses yet?
The Answer is… YES!
Companies like Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) are captivating investor interest right now and for good reason. While the hunt is on with traders scouring the web for any company with “coronavirus” or “COVID-19” as a keyword, Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) has begun rapidly expanding on its treatment platform.
Of course, there’s fundamental risk when investing at the early stages of any company, but there is also the potential of GREAT REWARD as we’ve clearly seen!
Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) is a clinical-stage pharmaceutical development company. Its lead compound is a repurposed drug called NP-120 (Ifenprodil). The orally delivered small molecule was originally developed by Sanofi to treat peripheral circulatory disorders.
In one of Algernon’s research programs, Ifenprodil showed promise as a possible treatment for idiopathic pulmonary fibrosis (IPF) and chronic cough. But things completely changed when it was discovered that the drug was effective against the world’s most lethal flu virus H5N1. When tested in an animal study, it reduced mortality by 40%, reduced acute lung injury (ALI) and reduced inflammation in the lungs.
[Read More] Best Penny Stocks To Buy Right Now? 3 For Your May 2021 Watch List
Ifenprodil was also shown in a separate animal study to prolong survival under anoxic (low oxygen) conditions, as might occur in patients with severely impaired lung function.
The connection was simple…if Ifenprodil works that well with H5N1, a virus that has a 50% mortality rate, how effective could it be for COVID-19, which has a 3% fatality rate?
Here’s Why Timing Could Be So Important Right Now
In early July, Algernon began screening patients for suitability for enrollment in its Phase 2 idiopathic pulmonary fibrosis (IPF) and chronic cough clinical study of its re-purposed drug NP-120 (Ifenprodil). Heading into August, Algernon upped the ante. The company has dosed its first patient in its Phase 2 IPF and chronic cough clinical study of its re-purposed drug NP-120 (Ifenprodil). The patient was enrolled and dosed at the Waikato Hospital located in Hampton, New Zealand.
But there’s more than just this trial at play. Algernon also received ethics approval from a central institutional review board for U.S. study sites for its multinational Phase 2b/3 human study of NP-120 (Ifenprodil) for COVID-19. This has lead to further developments in North America including the first U.S. Clinical Trial site in Florida.
Algernon completed its clinical trial agreement with Westchester Research Center at Westchester General Hospital in Miami, Florida, for its multinational Phase 2b/3 human study. “Of the 5 U.S. research institutions we have been working with, two are located in Florida where they have recently had a significant number of confirmed COVID-19 cases,” said Christopher J. Moreau.
What’s more is that Algernon’s drug candidate may already have started receiving validation by industry peers. An independent research study from UT Dallas’ Center for Advanced Pain Studies identified Ifenprodil as one of a number of possible drug candidates for the treatment of COVID -19. The Study was published online in Brain, Behaviour, and Immunity.
The Study identified interactions between the immune system and nerves in the lungs that can cause rapid deterioration in COVID-19 patients. The authors looked at the relative gene abundance in the lungs of COVID-19 patients compared to the lungs of healthy controls. In addition to genes that might be expected to be upregulated, the levels of the gene that expressed the NMDA GluN2B receptor in immune cells were also greatly increased.
“We were really taken aback at the upregulation of NMDA receptors in severe COVID-19 patients. We were even more surprised, when we noted that it was an immune based signal and not neuronal. Based on our findings, we think there is significant opportunity in disrupting NMDA receptor signalling in the lung for reversing pathology in COVID.”
Dr. Theodore Price, Co-Author of the independent research study
Algernon Could Stand Toe To Toe With Roche, Merck & More
If this wasn’t enough, background studies that Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) previously conducted showed that Ifenprodil outperformed the world’s leading two treatments for IPF:
Boehringer Ingelheim’s Nintedanib; and
Roche’s Pirfenidone
These were done in a pre-clinical in vivo animal study, reducing fibrosis by 56% with statistical significance. Algernon additionally investigated Ifenprodil in an acute cough animal study where it outperformed Merck’s phase 3 drug Gefapixant by 110%. Since Ifenprodil is already approved with an established safety history, Algernon intends to move the drug directly into a phase II human trial.
The Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) business model is to repurpose safe approved drugs that are not available in the US or Europe, screen them in globally accepted animal models for new diseases, file new intellectual property rights and then move them into an off label phase II trial in the country where they were originally approved. Once a signal is established in a human trial, the company will begin to advance the repurposed drug through a USFDA registration.
Is Ifenprodil The Silver Bullet?
Since Ifenprodil’s performance in H5N1 research studies was first discovered, Algernon has been aggressively engaged in advancing it as a potential new therapeutic for COVID-19 patients. The company has already filed a pre-IND (Investigational New Drug) meeting request with the U.S. FDA for the treatment and prevention of ALI and acute respiratory distress syndrome (ARDS) associated with COVID-19 infection. The Company believes that Ifenprodil may reduce the severity and duration of a COVID-19 infection. And in mid-April, the company formally received positive feedback from the U.S. FDA for Ifenprodil.

The company has already received positive feedback from Health Canada on its plan to conduct a phase 2 COVID-19 clinical study in Canada. Furthermore, Algernon has received a No Objection Letter from Health Canada to proceed with a NP-120 (Ifenprodil) COVID-19 Phase 2b/3 multinational clinical trial. In light of this, Algernon has now enrolled new patients into its trial. At the beginning of September, the company had officially enrolled 50 patients.
In addition to advancing Ifenprodil towards a human trial approval in Canada, the company has also received approval from the Ministry of Food and Drug Safety in South Korea, as well as ethics approval, for an investigator-led, Phase 2 COVID-19 clinical study of its re-purposed drug NP-120 (Ifenprodil), an NMDA receptor antagonist. This 40-patient trial is designed to test the effect of Ifenprodil in COVID-19 infected patients with severe pneumonia.
Furthermore, in August, a Miami hospital is the first in the country to test a possible COVID-19 treatment on humans. That treatment: Ifenprodil. The research center at Westchester General Hospital in Coral Terrace is on its way to enroll patients to test Algernon’s drug. As the company conducts testing worldwide, Westechester was the first site to be activated in Florida and will be investing $20,000 to $25,000 in the testing process.
Download / View
(OTC: AGNPF) (CSE: AGN)
Corporate Presentation, Below
Why Algernon Pharmaceuticals Inc.
(OTC: AGNPF) (CSE: AGN)
- Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) is one of the few companies still flying under the radar of the masses, potentially giving one of the last “ground floor” looks at a coronavirus stock before a pending breakout
- Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) already has a proven drug that has a strong safety history and was developed by one of the top names in biotech, Sanofi.
- Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) is advancing Ifenprodil into multiple phase 2 human trials in Australia, Canada, and South Korea.
- Algernon Pharmaceuticals Inc. (OTC: AGNPF) (CSE: AGN) has just announced the first patient dosed in Phase 2 IPF & Chronic cough human trial of Ifenprodil.
- While early names have gone to trade much higher, the next round of coronavirus stocks could be offering immediate opportunity, now.

End Notes:
– https://www.utdallas.edu/news/healthmedicine/nerve-cells-lungs-covid-19-2020/
– https://linkinghub.elsevier.com/retrieve/pii/S088915912030670X